Over the years, clinical research and development has transformed the face of disease management. It has provided critical information that facilitates decision making for policy makers, regulators, product developers, and insurers within the health sector. However, the success of any clinical research relies on correct generation, and collection and analysis of clinical trial data. Further, global health regulators are increasingly demanding for quality data handling processes before allowing new pharmaceutical products and medical devices into the market.
Consequently, Contract Research Organisations (CROs), pharmaceutical companies, life sciences organisations and other industry players have embraced new technologies to improve the quality of their data. Solutions such as clinical trial management software and many other innovations have now become an integral part of today’s clinical medicine development. Additionally, stakeholders are working to implement advanced solutions to meet the ever-changing regulatory requirements, as well as respond to technology growth. This article explores how technology has influenced clinical trial data management and its future effects.
Modern Clinical Trial Management Overview
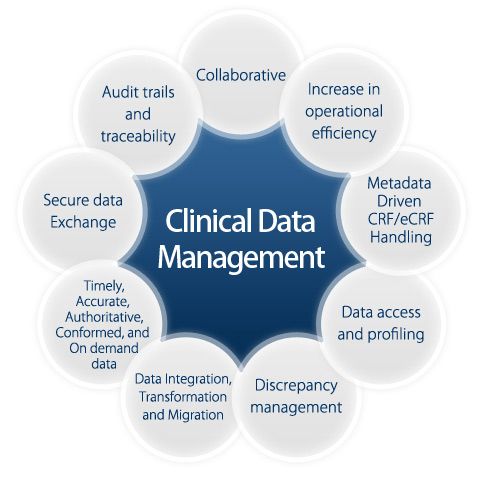
Clinical Trial Management (CTM) plays a critical role in clinical research, ensuring generation of quality, consistent and statistically relevant data from clinical trials. It takes the centre of every stage of a clinical trial from inception to completion. The efficiency of modern CTM solutions has significantly reduced the product development period, leading to faster commercialization of medical products. Today, CROs and sponsors use advanced technology to implement various trial procedure like data collection, validation, medical coding, discrepancy management and data protection among others. This helps research organizations to maintain quality standards across the clinical trial.
Drivers of clinical trial management software development
Challenges, legislation and technology growth are the major internal and external forces that drive the development of clinical trial management solutions. The following are some of the factors that have transformed CTM:
- The pressure within the clinical research field – Medical research centres have been under increasing pressure to minimize rising healthcare costs and to improve care quality. This requires research firms to maintain the quality of processes and data throughout the clinical trial. This creates the need for the development of IT systems that help pharmaceutical and biotechnology companies to improve the quality of products, shorten the development time, as well as comply with industry guidelines.
- Global regulations – Health regulators have been working to create a suitable environment for clinical trials while maintaining the safety of participants and the transparency of trial information. Moreover, in most jurisdiction, governments support the adoption of Patient Registry Database Software to facilitate post-marketing surveillance and provision of value-based care.
- Demand for integrated health solutions – Clinical Trial Management Software has been closing the gap between clinical research and the existing health system. Modern Hospital Information Systems (HIS) integrates with CTM solutions to enable accurate documentation process. This integration, together with the increasing use of e-Health records (EHRs) is fostering the growth of CTM software development.
- Availability of new technology – Technology has been growing at a rapid rate, disrupting almost every sector of the economy. The penetrations of smartphones, internet connectivity and many other state-of-art innovations have simplified clinical trial procedure as well as how trial participants send and receive information. Moreover, today’s CTM uses advanced technologies including machine learning and AI to enhance the speed, accuracy and the quality of trial procedures.
Major Benefits of CTM Solutions
Adoption of CTMS and Patient Registry Database Software in clinical trials provides a range of functionalities to track and manage protocols, subjects, billing and reporting among other components of clinical trials. The solutions centralize components of a particular study and incorporate valuable insights from different sources. This enhances trial productivity and participating patient experience, as well as increases the amount and quality of trial data. Currently, CTM offers a number of benefits to researchers and other teams involved in clinical trials:
- Improved Data Management
CTM systems provide a secure and central location for recording and storing trial activities, presenting a single-click retrieval of important information. The Patient Registry Database Software stores patient details, services and study documents, allowing multi-format data export and configuration of data entry forms. Besides, modern CTMS supports the generation of detailed reports and individual/multiple trials check as well as organizes information for sharing.
The system also enhances data security through user authentication and privileges to prevent unauthorized access or modifications. This ensures data integrity and compliance with regulators such as HIPPA, FDA, and the European GDPR. The efficiency of data management in a clinical trial further offers prompt insights into research operations.
- Clinical Trial Budgeting and Cost Reduction
Clinical Trials Management Software provides advanced features for estimating the costs of a study before its initiation. This can help researchers to control trial activities such as patient recruitment to save costs. The system supports consistent budgeting by tracking financial data to facilitate accurate invoicing and timely payment. Moreover, CTMS tracks anticipated revenue, simplifies estimation and monitoring of study budgets.
- Billing Compliance
CTM solutions support study calendar sharing, standardization, and centralization of billing processes to enhance compliance. It streamlines communication between clinical research units and integration with EMR systems to communicate real-time patient information from the clinic. This improves the billing accuracy and routing of study charges.
- Enhanced Clinical Trial Efficiency
Clinical trial data management supports an array of processes at various stages of a trial minimizing redundant data entry. Additionally, the system supports information sharing across various clinical research group, saving time as well as increases data consistency and accuracy.
- Managing Regulatory Compliance
A good clinical trial management software provides features for tracking industry regulations. This involves monitoring amendments, procedure and subject deviations among other industry guidelines to ensure compliance.
- Emerging Technology and the Future of Clinical Trial Data Management
Patient Registry Database Software development and many other CTMS integrations have increased the amount and complexity of clinical trial data. This calls for the invention of more advanced approaches to address the current challenges. Fortunately, researchers can invest in emerging technologies to incorporate insights from multiple data source to improve the quality of trial results. Emerging technologies provide opportunities for:
- Engagement – Enhancing the level of engagement with investigators and patients using real-time and targeted channels build strong relationships to meet the industry demands
- Efficiency – Digitization and rationalization of processes to optimize clinical trial time and costs.
- Product development – Innovation will speed up product and service development creating value for caregivers, patients, and sponsors. This will be possible by the use of digital endpoints, telemedicine, and other advanced platforms.
Transformative Technologies in the Future of Clinical Trial Management
IoT – Internet of Things can be used to collect data from the subject’s environment using a variety of objects such as biosensors. This can increase the volume, relevance and accuracy collection of trial data.
- Machine Learning – Machine learning can automate some of the clinical trial procedures, helping to reduce product development time.
- Artificial Intelligence – Particularly, AI can make can link with Patience Registry Database software and other knowledge bases to help match subjects to the right studies to minimize delays.
- Big Data Analytics – With the trial data increasing in both volume and complexity, big data analytics will help improve the data processing speed. Researchers will need to set up data centres and use cloud data management and storage tools to ensure efficiency and reduce data management costs.
- Mobile Apps – Today, people can do almost anything from their smartphones. CROs should invest in building clinical trial management app to increase data access and collaboration.
- Blockchain – The cryptocurrency technology will play a big role in CTM. Research companies can use blockchains to enhance data integrity and privacy. Blockchain will also ensure anonymity of shared data and allow access to the only authorized party, increasing regulatory compliance.
Summary
Clinical trial management has improved data quality and accessibility, facilitating quicker development of innovative products and services. However, CROs, sponsors and other industry players still face several challenges resulting from increasing complexity and amount of data as well as the emerging regulation. To improve the productivity of clinical trials and participators’ experience, researchers need to invest in emerging technologies such as AI, Blockchain, Mobile Apps and IoTs among others to build more advanced CTMS. This will address the current challenges and support innovation of accurate products, contributing to public health development.
Contributed byhttp://www.eclinicaltrials.co/